During contemporary rigorous factory environment, maintaining manufacture freshness stands as crucial. Automated pipeline sanitation systems have emerged as a integral strategy for guaranteeing spotless situations inside transfer lines. Pigging systems effectively prevent the transfer of residues between different product batches, guaranteeing product consistency and meeting stringent industry standards.
- In addition, implementing a system with smooth internal surfaces can markedly mitigate the aggregation of product residues.
- Applying pigging systems that support thorough cleaning and antisepsis is also critical.
Economic Manual Pigging Solutions
Manual pigging systems offer a effective and low-cost method for cleaning pipelines. These systems utilize hand-operated gadgets to expel buildup within conduits. While machine-driven pigging systems offer greater rapidness, manual systems provide a suitable alternative for infrequent cleaning tasks.
- Strengths
- Cost-effectiveness
- Straightforward Operation
Manual pigging systems usually necessitate skilled operators to deploy the pigs within the pipeline. These staff oversee the mechanism's progress and ensure the successful cleaning of buildup.
Key Perks of Hygienic Pigging in Food Factories
Hygienic pigging affords a range of significant advantages within the food processing industry. It effectively eliminates the risk of cross-contamination by thoroughly cleaning pipelines and equipment with specialized pigs. This improves product safety, meeting stringent food safety regulations. Furthermore, hygienic pigging reduces downtime for cleaning and sanitation processes, increasing operational efficiency.
- Furthermore, it lengthens the lifespan of equipment by preventing corrosion and buildup.
- Hence, hygienic pigging contributes to a healthier production environment, safeguarding both product quality and consumer well-being.
Manual Pigging Solutions: Choosing the Right Option
When it comes to pigging operations, selecting between an automated and a manual system can be a crucial decision for pipeline maintenance. Both approaches offer distinct advantages and disadvantages that should be carefully weighed against your particular operational needs. Manual pigging systems often require physical presence, while automated systems utilize advanced technology to track the process remotely. Factors such as line size, frequency of cleaning, and budget constraints can all play a role in determining the most suitable pigging system for your application.
- Automated systems often provide increased efficiency, reducing downtime and labor demands.
- However, manual pigging systems may be more budget-friendly for sporadic use or smaller pipelines.
Ultimately, the best choice depends on a thorough assessment of your unique situation.
Dairy Farming and Hygienic Pigging Practices
This case study/analysis/investigation focuses on the successful implementation/adoption/integration of a hygienic pigging system within a dairy farm/milk processing facility/large-scale dairy operation. The goal/aim/objective of this project was to enhance/improve/optimize sanitation practices and reduce/minimize/decrease contamination risks throughout the production/processing/handling pipeline. By utilizing/employing/leveraging a multi-stage/comprehensive/sophisticated pigging system, the dairy operation/farm/facility achieved significant improvements/gains/successes in hygiene standards, resulting/leading to/causing a noticeable reduction/decrease/decline in product contamination/spoilage/rejection rates.
- Key findings/Notable results/Major takeaways from this case study/analysis/investigation include/highlight/demonstrate the effectiveness/efficiency/benefits of a well-designed hygienic pigging system in maintaining food safety/product quality/hygienic standards.
- Furthermore/Additionally/Moreover, the implementation/adoption/integration of this system contributed/aided/facilitated to increased efficiency/process optimization/reduced downtime within the dairy operation/farm/facility.
This study/case/analysis serves as a valuable resource/guideline/example for other dairy producers/industry stakeholders/farms looking to improve/enhance/optimize their sanitation practices and ensure/guarantee/maintain the highest levels of hygiene/standards of quality/safety protocols.
Pipeline Integrity in the Pharmaceutical Industry: Ensuring Adherence to Regulations
Pharmaceutical manufacturers need meticulous procedures for product transfer and line cleaning. Pigging systems, a necessary component in this process, offer a strong means to guarantee sterility and reduce cross-contamination between batches. Compliance bodies impose strict guidelines on pharmaceutical production, necessitating manufacturers to abide by rigorous directives for pigging system operation and maintenance. This involves meticulous documentation, regular assessments, and training of personnel. Adherence to these rules is paramount not only for product quality but also for defending public health.
To guarantee compliance, pharmaceutical companies have no choice but to implement a comprehensive pigging system scheme that encompasses every component of the process, from deployment and carrying out to maintenance and record keeping. This contains identifying pigging systems suited to specific product characteristics, building standardized operating procedures (SOPs), and conducting scheduled system verification. Also, ongoing coaching programs for personnel are indispensable to support a high level of obedience and foster best practices.
By using a robust pigging system strategy and nurturing a culture of compliance, pharmaceutical manufacturers can productively mitigate risks, safeguard product integrity, and achieve regulatory standards.
Guidelines for Maintaining a Hygienic Pigging System
Maintaining a hygienic pigging system is necessary for promoting product condition. Regular upkeep can restrain contamination and confirm the trustworthy transfer of items. A all-encompassing pigging system maintenance program ought to entail a bundle of actions including consistent evaluations, breakdown for deep cleaning, and the renewal of worn parts. Embracing these best practices is likely to maximize the operational duration of your pigging system and guarantee a sterile operation.
- Keep to manufacturer guidelines for cleaning and maintenance.
- Use appropriate compounds and methods to effectively expel contamination.
- Document all maintenance works for future audit.
Pigging Systems
In the world of pipeline maintenance, pigging systems are an essential tool for ensuring optimal functionality . These ingenious devices utilize specialized tools called pigs to transit through pipelines, effectively eliminating debris and buildup. Understanding the diverse types of pigging systems is crucial for selecting the most appropriate solution for your specific needs.
- Physical pigs utilize force to dislodge obstructions , while gel pigs employ chemical solutions to dissolve buildup. Controlled pigs are used for tasks like inspection pipeline integrity, while smart pigs can gather information on pipeline conditions.
Regardless of the specific type, pigging systems offer a cost-effective and efficient method for ensuring the health and longevity of your pipelines.
Hygienic Pigging: Emerging Innovations
The pigging realm is poised for significant evolutions with a focus on hygiene. As consumers require ever-higher levels of food safety and product excellence, hygienic pigging technology will become increasingly important. Advanced technologies are in development to enhance the efficiency of cleaning and sanitation processes within pipelines. Advanced pigging systems, coupled with bio-compatible components, will play a key role in minimizing impurity. This transformation towards hygienic pigging promises to create a more secure environment for food processing and other fields that rely on pipeline transportation.
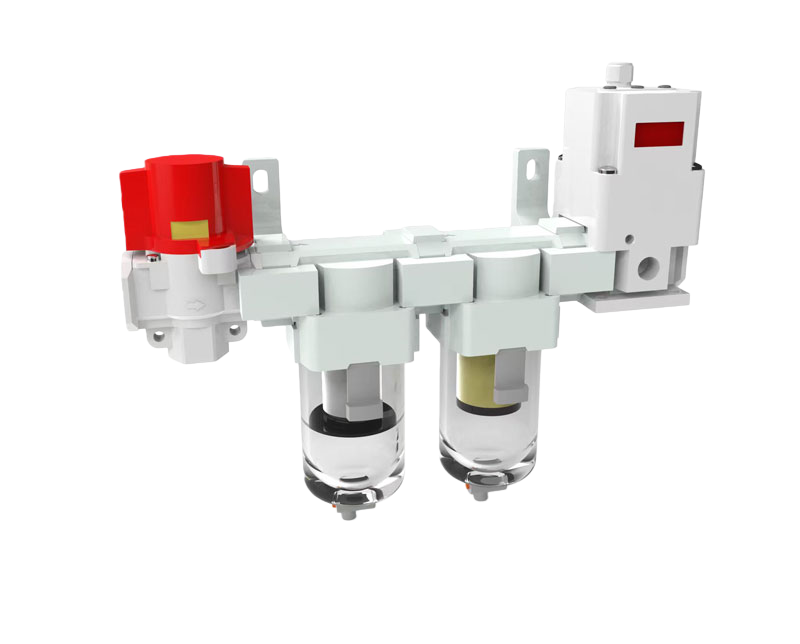 manual pigging system
manual pigging system